Significance of Transformations Lines
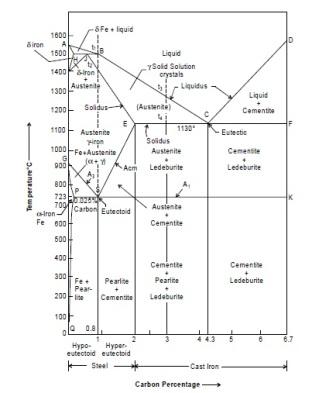
Line ABCD
The line ABCD tells that above this line melting has been completed during heating the iron. The molten metal is purely in the liquidus form. Below this line and above line AHJECF the metal is partially solid and partially liquid. The solid metal is known as austenite. Thus the line ABCD represents temperatures at which melting is considered as completed. Beyond this line metal is totally in molten state. It is not a horizontal line the melting temperature will vary with carbon content.
Line AHJECF
This line tells us that metal starts melting at this temperature. This line is not horizontal and hence the melting temperatures will change with carbon content. Below this line and above line GSEC, the metal is in solid form and having austenite structure.
Line PSK
This line occurs near 723°C and is a horizontal line and is known as lower critical temperature line because transformation of steels starts at, this line. Carbon % has not effect on it that means steel having different % of carbon will transforms at the same temperature.
The range above the line up to GSE is known as transformation range. This line tells us the steel having carbon up to 0.8% up to 0.8% will starts transforming from ferrite and pearlite to austenite during heating.
Line ECF
It is a line at temperature 1130°C which tells that for cast iron having % of C from 2% to 4.3%. Below this line and above line SK, Cast iron will have austenite + ledeburite and cementite + ledeburite. Copied from Introduction to Basic Manufacturing Processes and Workshop Technology by Rajender Singh. If you want to learn about anything related to technical areas then visit this page www.engineersgallery.com.
Click here to see different engineering project in the final year of engineering as well as after the engineering study.







Post Comment
You must be logged in to post a comment.